Oxidative phosphorylation, the final stage of cellular respiration, stands as a masterpiece of energy conversion. Let's embark on a journey into this remarkable process, where electrons dance along a chain, protons flow like a river, and ATP, the energy currency of life, is minted in abundance.
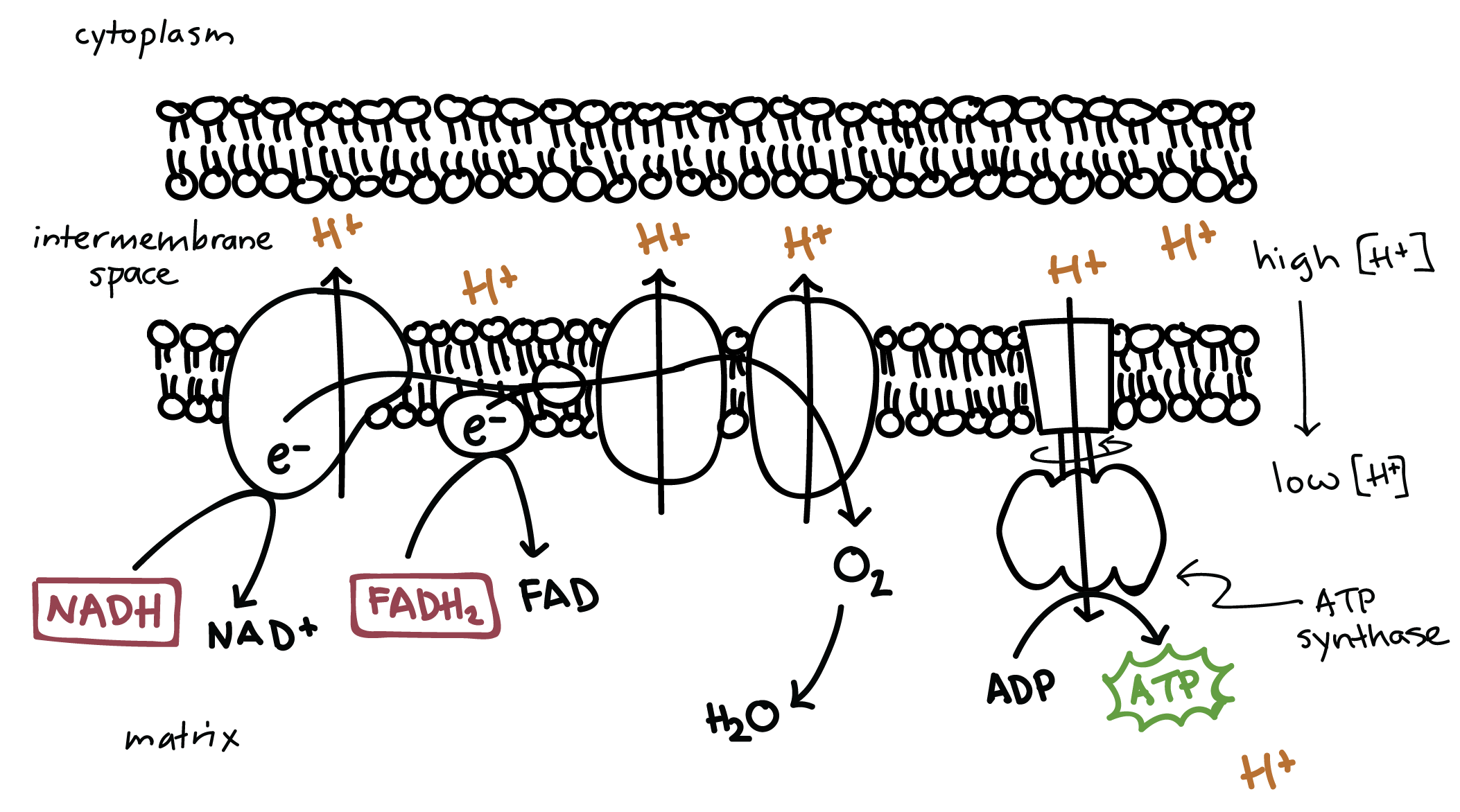
At the heart of oxidative phosphorylation lies the electron transport chain (ETC), a series of protein complexes embedded in the inner mitochondrial membrane. This chain functions as a molecular conveyor belt, passing electrons from one complex to the next. The electrons originate from the energy-rich molecules NADH and FADH2, generated in earlier stages of cellular respiration (glycolysis and the citric acid cycle).
As electrons move along the ETC, they release energy in a controlled manner. This energy is used to pump protons (H+) from the mitochondrial matrix to the intermembrane space, creating an electrochemical gradient across the inner membrane.
ATP Synthase: The Molecular Turbine
The Yield: A Bounty of ATP
The Role of Oxygen: The Final Electron Acceptor
Beyond ATP: Generating Heat
Conclusion
At the heart of oxidative phosphorylation lies the electron transport chain (ETC), a series of protein complexes embedded in the inner mitochondrial membrane. This chain functions as a molecular conveyor belt, passing electrons from one complex to the next. The electrons originate from the energy-rich molecules NADH and FADH2, generated in earlier stages of cellular respiration (glycolysis and the citric acid cycle).
As electrons move along the ETC, they release energy in a controlled manner. This energy is used to pump protons (H+) from the mitochondrial matrix to the intermembrane space, creating an electrochemical gradient across the inner membrane.
ATP Synthase: The Molecular Turbine
This proton gradient, also known as the proton motive force, is a reservoir of potential energy. It's harnessed by a remarkable enzyme complex called ATP synthase, a molecular turbine that spans the inner mitochondrial membrane. As protons flow back into the matrix through ATP synthase, they drive the rotation of a protein rotor, which in turn facilitates the synthesis of ATP from ADP (adenosine diphosphate) and inorganic phosphate.
 |
| ATP |
The Yield: A Bounty of ATP
Oxidative phosphorylation is the most efficient stage of cellular respiration, yielding the vast majority of ATP produced from glucose. While the exact number can vary depending on cellular conditions, approximately 32-34 ATP molecules are generated per glucose molecule through oxidative phosphorylation.
The Role of Oxygen: The Final Electron Acceptor
Oxygen is essential for oxidative phosphorylation. It serves as the final electron acceptor at the end of the ETC. When oxygen accepts electrons, it combines with protons to form water (H2O). Without oxygen, the ETC would become backed up, and ATP production would cease, leading to cell death.
 |
| Electron Acceptor |
Beyond ATP: Generating Heat
While ATP is the primary product of oxidative phosphorylation, not all the energy is captured in this form. Some of it is released as heat, contributing to the maintenance of body temperature in warm-blooded animals.
Conclusion
Oxidative phosphorylation is a testament to the elegance and efficiency of cellular energy conversion. It's a complex yet remarkably efficient process that sustains life by providing a constant supply of ATP. As we continue to explore the intricate details of this process, we gain deeper insights into the fundamental principles that govern life's energy economy and open doors for potential therapeutic interventions in various diseases. The power of oxidative phosphorylation truly illuminates the wonders of life's biochemical machinery.